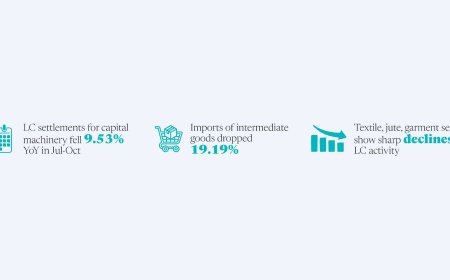
Govt excludes pharma representatives from drug regulatory bodies
The decision aims to curb corporate influence and rebuild public trust.
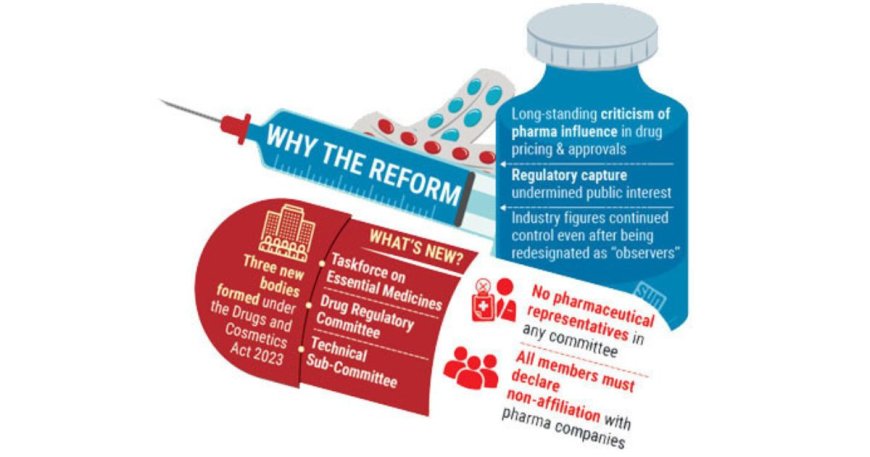
Govt Overhauls Drug Regulatory Committees, Removes Pharma Industry Ties
In a landmark effort to curb corporate influence and restore public confidence, the Bangladesh government has restructured its top drug regulatory bodies, eliminating pharmaceutical industry representatives for the first time in over three decades.
The interim government's decision comes amid mounting criticism that pharmaceutical companies have long exercised excessive control over key regulatory functions—ranging from drug approvals to pricing and safety monitoring. Observers say this dominance led to regulatory capture, where private interests consistently outweighed public health priorities.
Historically, pharmaceutical executives—first as official members, later rebranded as “observers”—held significant sway over decision-making processes within the drug oversight framework. Now, under the Drugs and Cosmetics Act 2023, the Ministry of Health has established three new regulatory bodies entirely free of industry affiliation: the Taskforce on Essential Medicines, the Drug Regulatory Committee, and a Technical Sub-Committee.
“These are the most consumer-focused drug committees we've seen in decades,” a senior Health Ministry official said anonymously. “We've intentionally excluded anyone with known conflicts of interest.”
Under the new rules, all committee members—and even alternates—must formally declare that they have no ties to pharmaceutical companies.
Long-Awaited Reform
“For years, health professionals have called for removing pharmaceutical representatives from regulatory roles,” said Professor Syed Abdul Hamid of Dhaka University’s Institute of Health Economics. “Having companies oversee their own products is a textbook case of conflict of interest. This reform is a crucial first step—but its success hinges on appointing independent and technically competent experts.”
He cautioned that removing vested interests alone won’t suffice. “Without integrity and expertise, political favouritism could simply replace corporate capture.”
The Health Ministry has described the new panels as “independent, academically grounded, and shielded from undue influence.”
Reversing Years of Industry Dominance
Experts say the pharmaceutical industry's grip on drug regulation tightened in the early 2000s.
“Prior to the 1982 National Drug Policy, public health protections were stronger,” said a senior official. “But after 2002, industry-linked figures gradually infiltrated key committees. By 2009, a handful of dominant firms held disproportionate sway over policy decisions.”
One industry insider reportedly directed pricing and regulatory agendas for over a decade. “He was never dismissed—just reshuffled,” the official added.
New Committees and Their Composition
The newly formed Taskforce on Essential Medicines will be chaired by Bangladesh Medical University (BMU) Vice-Chancellor Prof Dr Md Shahinul Alam. The Ministry of Health’s Joint Secretary Mohammad Mozammel Hossain Khan will serve as member secretary. The 16-member panel includes pediatricians, pharmacologists, economists, and academics—without any industry representation.
The Drug Regulatory Committee, chaired by the Health Secretary, will include members from the Pharmacy Council, Bangladesh Medical Association (BMA), Dhaka University’s Pharmacy and Clinical Pharmacology departments, and representatives from medical colleges. The Director General of the Directorate General of Drug Administration (DGDA) will serve as member secretary.
The Technical Sub-Committee, led by the DG of DGDA, will comprise experts from the Armed Forces Medical College, the Institute of Epidemiology, Disease Control and Research (IEDCR), BMU, and various professional medical societies.
Toward a Transparent, Science-Based System
Public health experts have widely welcomed the overhaul, calling it a critical step toward depoliticising and professionalising Bangladesh’s drug regulatory framework.
“This is an opportunity to build a transparent, science-driven regulatory system,” said a former health adviser to the government. “But long-term success will depend on continued political commitment, openness, and accountability to the public.”
What's Your Reaction?